Research groups
HCEMM-USZ Cerebral Blood Flow and Metabolism Research Group
Continuous, undisturbed blood supply to the brain is essential to optimal neural function. The brain accounts for 2% of the body mass, yet it receives 15% of the cardiac output and consumes 20% of the full body's oxygen supply. Any reduction or a very brief cessation of cerebral blood flow has, therefore, a detrimental impact on brain function. The goal of our research is to understand the cellular mechanisms of neuronal injury in acute cerebrovascular disorders.
Our ongoing research focuses on ischemic stroke. The development of effective strategies to limit the progression of secondary injury is of fundamental importance to improve the prospect of successful recovery. As a first step, it is essential to recognize distinct injurious phenomena that evolve over the subacute and chronic phases of ischemic brain injury (e.g. brain edema, vasospasm, oxidative stress, excitotoxicity). The phenomenon known as spreading depolarization is a central mechanism of secondary ischemic brain injury. We explore the signaling pathways set in motion by spreading depolarizations. We also study the impact of aging on the progression of ischemic brain injury. This is a timely and pertinent area of research since the incidence of ischemic stroke increases exponentially after the age of 50 years. Our work is essentially translational; the data generated is expected to be turned into the design of effective therapies to alleviate stroke symptoms.
Members:
- Prof. Dr. Eszter Farkas
- Dr. Ákos Menyhárt
- Dr. Szilvia V. Kecskés
- Dr. Rita Frank
- Dr. Anna Törteli
- Dr. István Pesti
- Petra Somogyi
Gut–Brain Axis Research Group

The enteric nervous system (ENS), located within the gastrointestinal tract, is aptly called our “little brain”: it is capable of complex, autonomous functions even without input from the central nervous system. In humans, it contains hundreds of millions of neurons, comparable in number to those of the spinal cord. Although it is often referred to as the “second brain,” from an evolutionary perspective it is rather the “first,” since it is already present in brainless organisms such as hydras. As the conductor of the gastrointestinal tract, the ENS regulates mucosal secretion, permeability, blood flow, and motility, while maintaining constant communication with the brain. About 70–80% of our immune cells reside in the gut, which tightly links ENS function to the immune system and to the gut microbiota. In recent years, the gut–brain axis has been implicated in several central nervous system disorders, including stroke and Alzheimer’s disease. Our research focuses on the physiological and pathological functions of the enteric nervous system and on diseases arising from disturbances in gut–brain interactions. In our experiments, we investigate the role of the ENS and its interactions in common conditions such as functional dyspepsia, irritable bowel syndrome, and acute ischemic stroke. Our goal is to translate our research findings into direct benefits for patients through potential new therapies and diagnostic approaches.
Members:
Neuroinflammation Research Group
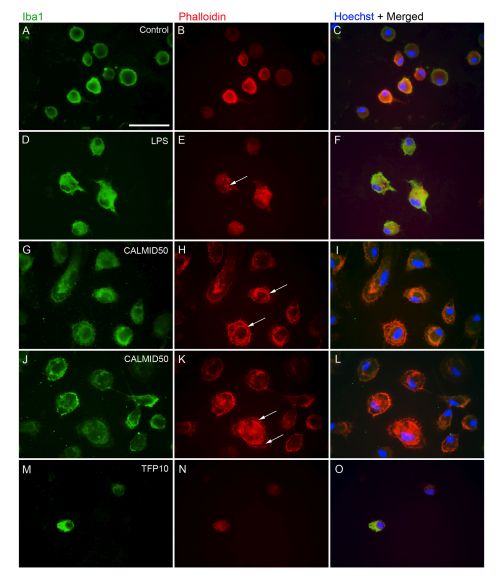 Different activated and ramified microglia populations in embryonic- or newborn derived primary cortical cultures can be separated with different morphological, molecular, immuncytochemical and functional (proliferation and phagocytosis capacity) methods. In our study, we explore the action of anti- and proinflammatory compounds on the re-arrangement of cytoskeletal actin in microglial cells.
Different activated and ramified microglia populations in embryonic- or newborn derived primary cortical cultures can be separated with different morphological, molecular, immuncytochemical and functional (proliferation and phagocytosis capacity) methods. In our study, we explore the action of anti- and proinflammatory compounds on the re-arrangement of cytoskeletal actin in microglial cells.
We apply in our in vitro system morphological and functional assays (cell proliferation and viability, phagocytosis, motility test and several gene expression measurement-based methods). Beside the examination of transcriptional and epigenetic regulation of the pro-and anti-inflammatory gene expression, we also analyze specific intracellular signalization pathways potentially implicated in the activation of the microglia.
We manipulate environmental factors in the cultures (temperature, inflammatory and osmotic factors) in combination with the application of inhibitors of actin cell cortex re-arrangement, or modulators of inflammatory process.
Our in vitro experiments are used to replicate various aspects of neuroinflammation, neurodegeneration and regeneration (typical of Alzheimer’s or Huntington disease) and are expected to contribute to the better understanding of pro- and anti-inflammatory signaling in microglia. Our work contributes to a multidisciplinary research panel, in which the dysfunction of cellular signaling in the nervous tissue is studied to aid the development of effective therapeutic strategies for the treatment of neurodegenerative disorders.
Members:


